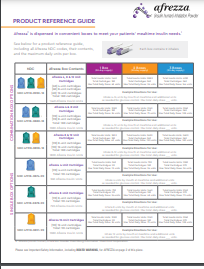
Access and Support Made Easier.
AfrezzaAssist® services include:
- Simplifying the prior authorization process
- Pharmacy fulfillment
- Financial assistance programs for eligible patients
- Customer support by phone
To ensure your patients can access these options, please submit prescriptions to
Sterling Pharmacy, the AfrezzaAssist® intake pharmacy.
Sterling Specialty Pharmacy
1312 Northland Drive Ste 500
Mendota Heights, MN 55120
NPI: 1225548480 eScribe: 2433693
HOURS: Monday – Friday, 7:30 AM – 5:30 PM CT
Accelerating access to Afrezza
AfrezzaAssist® provides free product to eligible patients who encounter
delays or restrictions while coverage is being pursued.
Actual Afrezza user
Getting Started With Afrezza®
US-AFR-2250